The iodine fluorine bonds are single bonds and the iodine oxygen bond is a double bond. The C - Cl bond in COCl2 is polar or nonpolar.
Solved Draw The Lewis Structure For Pf Including Lone Chegg Com
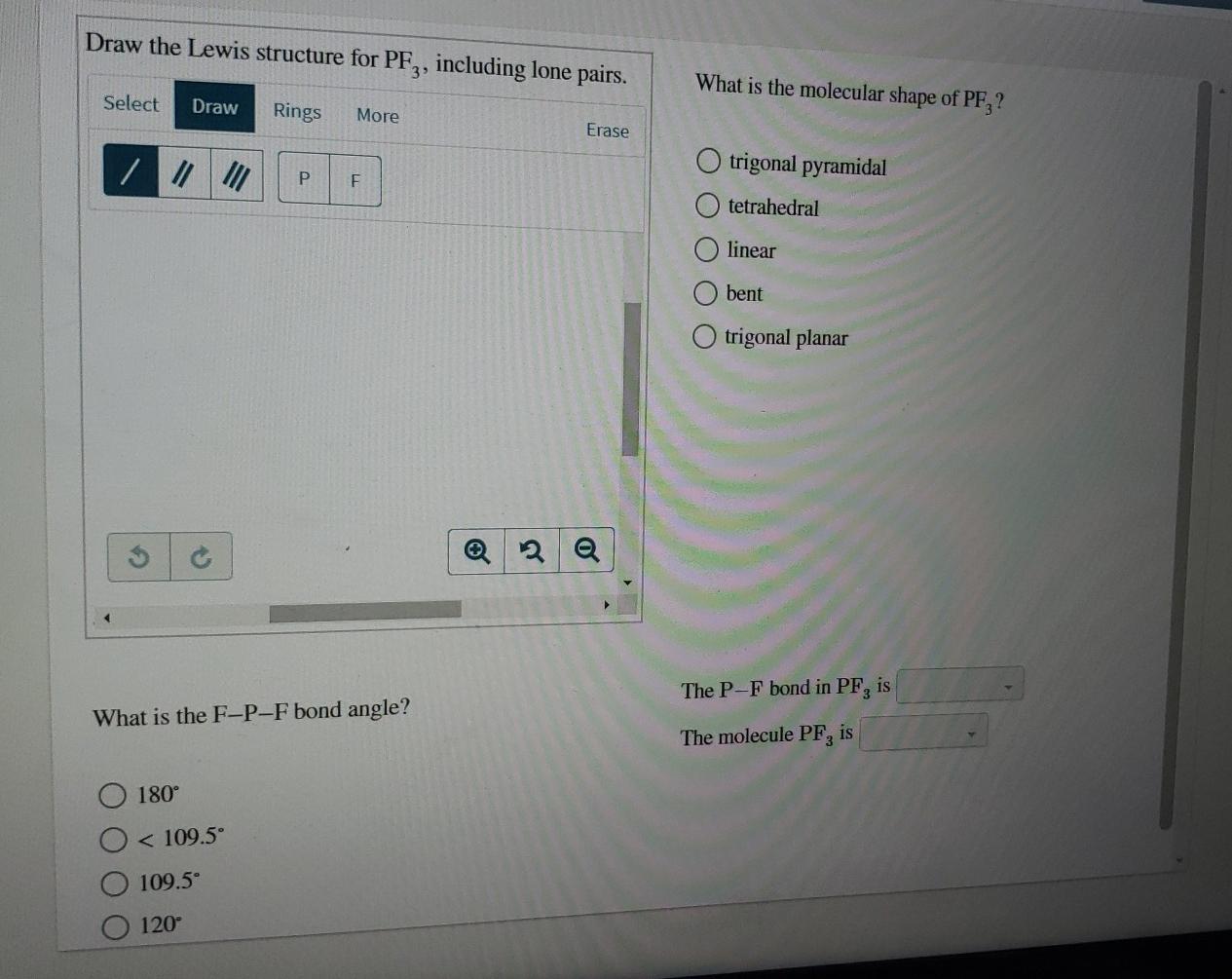
Draw the Lewis structure for PF3.

. This theory explains that the bond angle between the fluorine-phosphorus-fluorine F-P. Mark lone pairs Step 3. Lewis dot structure of PF3 contains 1 lone pair on the central.
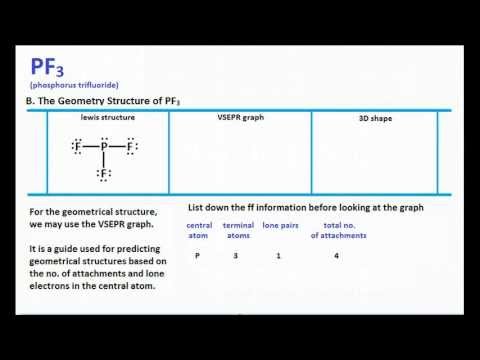
Geometrical Structure of Phosphorus Trifluoride PF3 molecule. Three Lewis structures are possible for N2O. Drawing the Lewis Structure for PF.
A central iodine atom is bonded to five fluorine atoms and one oxygen atom. Lewis dot structure of PF3 contains 1 lone pair on the central atom phosphorous and 3 lone pairs on each outer atom fluorine. Lastly draw the lewis diagram as.
Four regions of high electron density gives a tetrahedral structure The lone pair occupies more space. 3 bonding pairs and one lone pair or. Phosphorus has one single bond two double bonds and one lone pair of electrons.
A step-by-step explanation of how to draw the PF3 Lewis Dot Structure Phosphorous trifluorideFor the PF3 structure use the periodic table to find the tota. Draw the molecule by placing atoms on the grid and connecting them with bonds. 834 use lewis symbols and lewis structures diagram formation of pf3.
One fluorine has one single bond and three lone pairs of electrons. To add lone pairs click the button before clicking on the molecule. I am putting a photo below to help you alittle bit.
Draw sketch Step 2. What is the - 17371958. Three pairs will be used in the chemical bonds between the P and F.
Draw and complete Lewis Structure of C3H3N and C2H2N -1. May 21 2009. The electronic geometry therefore is tetrahedral 1 lone pair and 3 pairs of bonded electrons.
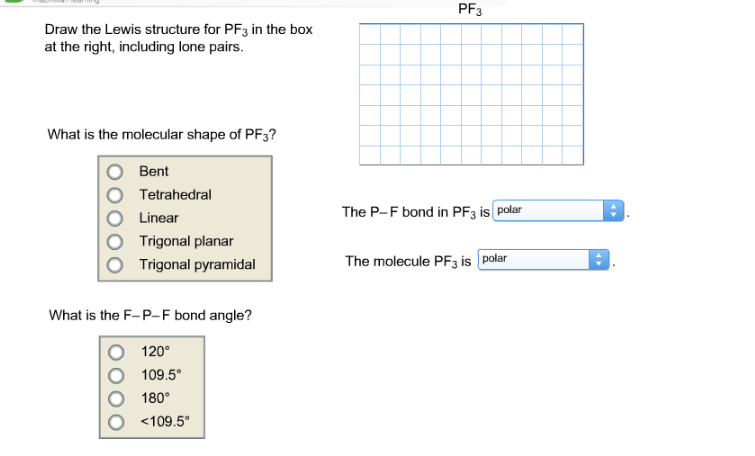
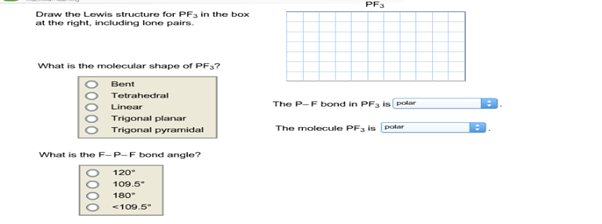
Each fluorine atom has three lone pairs of electrons and the oxygen atom has two lone pairs of electrons. Draw the Lewis structure for COCl2 including lone pairsWhat is the molecular shape of COCl2. PF3 Draw the Lewis structure for PF3 in the box at the right including lone pairs.
Two of the fluorines have one double bond and two lone pairs of electrons. B Draw the Lewis structure expected to be the second most important based on formal charges. Include lone pairs of electrons and.
O Bent O Tetrahedral O Linear O Trigonal planar The P-F bond in PF3 ispolar Trigonal Posted 6 months ago Number of Electron groups Molecular Geometry Around Central Atom and Name of Molecule Lewis Structure draw and state the name or nonpolar their Geometric Arrangement CH2Cl2 13T TeCl4 XeFs PF3 SeCl6. Draw the Lewis structure of PH3. The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density.
Be sure to show lone pairs and formal charges in the diagram. Mar 29 2022 0145 PM. NF3 Lewis structure wise looks much like PF3.
A Draw the Lewis structure expected to be the most important based on formal charges. Include all atomsbondslone pairs and formal charges. Heres how you can draw the PF 3 lewis structure step by step.
The molecular geometry or shape for PF3 is the trigonal pyramid. Therefore the tetrahedral bond angle of 109 o is reduced somewhat. The geometrical structure of the tetra-atomic Phosphorus Trifluoride PF3 molecule is studied with the help of the Valence Shell Electron Pair Repulsion VSEPR theory.
Be sure to show lone pairs and formal charges on the. EXPLAIN all your thought process. Which statement below best describes this Lewis structure.

Pf3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
Solved Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com

Pf3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
Get Answer Pf3 Draw The Lewis Structure For Pf3 In The Box At The Right Transtutors
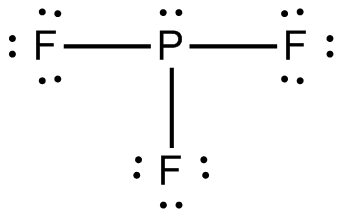
Compare The Structures Of So3 To Pf3 And Explain Why They Have Different Molecular Shapes Socratic
0 comments
Post a Comment